|
|
The Hodgson Group | |
|
|
Our research programme revolves around the theme of synthesis and encompasses broadly based studies directed towards the design and development of new methods, reagents and strategies for the synthesis of biologically active molecules. Specific research areas of current interest include the following:
One of the best ways of assembling five-membered heterocyclic rings with the potential for simultaneous generation of multiple chiral centres is by 1,3-dipolar cycloaddition reactions. The synthetic challenge in developing efficient catalytic asymmetric cycloaddition methodology in this area is considerable, and the field is still in its infancy. The aims of our research in this area are to build upon our recent results in asymmetric carbonyl ylide cycloadditions by evaluating and understanding the factors influencing asymmetric induction, with the goal of developing highly enantioselective processses for use in synthesis.
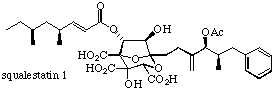
Squalestatin 1 is a fascinating complex molecule recently discovered during screening programmes of fungal metabolites to find inhibitors of specific enzymatic steps in the synthesis of cholesterol; it is the first natural product found to be a potent inhibitor of squalene synthase and it also exhibits broad spectrum antifungal activity. Because of its extraordinary potency squalestatin represents an exciting new therapeutic lead in the treatment of hypercholesterolemia and fungal infections. The scientific challenge in developing an efficient asymmetric entry to the squalestatins is formidable; our synthetic approach currently under investigation hinges on a cycloaddition-rearrangement strategy to rapidly construct the densely functionalised core.
Enantioselective desymmetrisation of achiral materials is an attractive and potentially extremely powerful concept in asymmetric synthesis. Achiral epoxides represent an important class of substrates which can be used to develop new desymmetrisation methodology because they are easily prepared with predictable stereochemistry directly from alkenes. We are examining a number of methods to achieve enantioselective desymmetrisation and are also applying the chemistry towards structurally and biologically interesting synthetic targets. We have found that desymmetrisation of cyclopentene oxides provides access in high enantiomeric excesses to cyclopentenols which can be used in the synthesis of anti-HIV agents such as carbovir, prostaglandins and the insecticidal iridoid (+)-iridomyrmecin. Another enantioselective method currently under investigation involves devising ways of discriminating between the removal of enantiotopic H-atoms on an epoxide ring. This process has the potential to create new ring systems with simultaneous generation of asymmetry.
Main group chemistry, especially involving silicon and tin, offers many opportunities for new reaction design. For example, we are evaluating consecutive chemoselective vinylstannane formation Pd-catalysed cross-coupling strategies in the synthesis of medium-rings. Here temporary 'binding posts' may overcome normally unfavourable entropy factors in such cyclisations reactions. A new entry to biologically active periplanones becomes viable on this basis.
|