|
|
The Hodgson Group | ||
|
Reviews
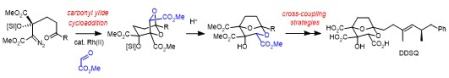
12) 'Evolution of a Cycloaddition-Rearrangement Approach to the Squalestatins: A Quarter-Century Odyssey'H. A. A. Almohseni and D. M. Hodgson, Synlett, 2020, 31,1555. 11) 'Recent Applications in
Natural Product Synthesis of Dihydrofuran and -Pyran Formation by Ring-Closing
Alkene Metathesis'
10) 'Utility of Unsaturated Terminal Epoxides in Cyclopropane Synthesis' D. M. Hodgson and S. Salik, Curr. Org. Chem., 2016, 20, 4. DOI:10.2174/1385272819666150601211502 9) 'Methods for Direct Generation of α-Alkyl-Substituted Aldehydes' D. M. Hodgson and A. Charlton, Tetrahedron, 2014, 70, 2207 (incl. Cover Article). DOI: 10.1016/j.tet.2013.11.046
8) 'Rich Chemistry of α-Lithiated Epoxides and Aziridines' D. M. Hodgson, Latvian J. Chem., 2009, 313. link
7) Synthesis of Azabicyclic Systems using Nitrogen-Directed Radical Rearrangements' D. M. Hodgson and L. H. Winning, Org. Biomol. Chem., 2007, 5, 3071 (incl. Cover Article).
6) Widening the Usefulness of Epoxides and Aziridines in Synthesis' D. M. Hodgson, P. G. Humphreys and S. P. Hughes, Pure Appl. Chem., 2007, 79, 269.
5) Expanding the Utility of Lithiated Epoxides and Aziridines in Synthesis' D. M. Hodgson, C. D. Bray and P. G. Humphreys, Synlett, 2006, 1.
4) Recent Developments in the Chemistry of Lithiated Epoxides' D. M. Hodgson and E. Gras, Synthesis, 2002, 1625.
3) Catalytic Enantioselective Rearrangements and Cycloadditions Involving Ylides from Diazo Compounds' D. M. Hodgson, F. Y. T. M. Pierard and P. A. Stupple, Chem. Soc. Rev., 2001, 30, 50. DOI: 10.1039/b000708k
2) Enantioselective Desymmetrisation of Achiral Epoxides' D. M. Hodgson, A. R. Gibbs and G. P. Lee, Tetrahedron, 1996, 52, 14361. DOI:10.1016/0040-4020(96)00888-5
1) Chromium(II)-Based Methods for Carbon-Carbon Bond Formation' D. M. Hodgson, J. Organomet. Chem., 1994, 476, 1. |